Parents Report Illness in Babies from ByHeart Formula Before Botulism Outbreak
Recent investigations by health officials have revealed over 30 cases of infant botulism associated with ByHeart baby formula, which emerged after August 2023. Families of infants who became ill months prior are seeking answers, as their experiences seem overlooked. Parents report that their babies faced similar illnesses as early as November 2022, prior to the current outbreak.
Cases Linked to ByHeart Baby Formula
California health officials confirmed that between November 2022 and June 2023, six infants who consumed ByHeart formula were treated for botulism. This was close to nine months before the recent surge in cases affecting at least 31 infants across 15 states. Despite recognizing these earlier cases, officials stated that their link to the present outbreak remains unconfirmed.
Parent Testimonies
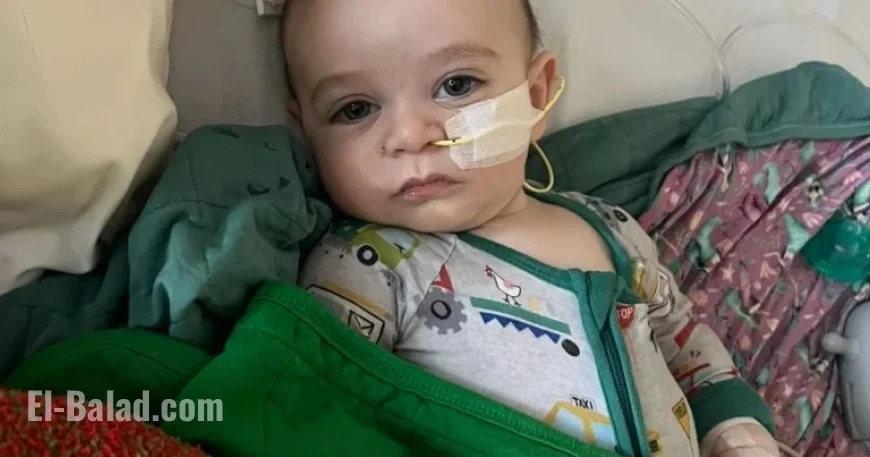
- Amy Mazziotti from Burbank reported her then-5-month-old son, Hank, was treated for botulism after consuming ByHeart formula in March.
- Katie Connolly from Lafayette stated her daughter, M.C., was hospitalized in April after being fed ByHeart formula.
Both mothers had been unaware of any potential source of their babies’ illnesses until the recall of ByHeart products on November 11, 2023. Mazziotti openly expressed suspicion regarding the correlation between ByHeart and the illnesses.
Laboratory Findings and Ongoing Investigation
Recent laboratory tests on unopened ByHeart formula revealed contamination with the bacteria that causes infant botulism. Attorney Bill Marler, representing the affected families, noted additional cases that predated the outbreak, including one infant who consumed the formula in December 2022 and others in spring 2023.
The CDC is aware of these earlier illnesses but currently focuses on understanding the spike in cases since August 1, 2023. Dr. Jennifer Cope from the CDC stated that this surge has provided stronger evidence linking ByHeart to the ongoing outbreak.
Concerns from Experts
Health officials indicated that the lack of documentation from parents may complicate efforts to connect these earlier cases to the outbreak. Connolly voiced her frustration over the perceived oversight of earlier cases, questioning why they were not investigated sooner.
Health professionals affirmed that prior to this outbreak, no powdered infant formula in the U.S. had tested positive for the botulism bacteria. Less than 200 cases of infant botulism are reported annually in the U.S., making these occurrences significant.
Concerns persist among experts about the accuracy of the CDC’s investigation regarding earlier cases. Frank Yiannas, a former FDA food policy deputy commissioner, emphasized that the earlier cases should be included in the investigation.
Impact on Families
Parents express deep concern for their infants’ health, which has been impacted by botulism. Symptoms typically include constipation, poor feeding, and weakness. Connolly and Mazziotti report that their children are recovering but continue to experience lingering effects.
As investigations continue, families demand transparency regarding the potential link between ByHeart formula and these serious health issues. Many parents believe they deserve answers about the safety of products fed to their newborns.