UK Launches Trial for Prostate Cancer Treatment with Reduced Side Effects
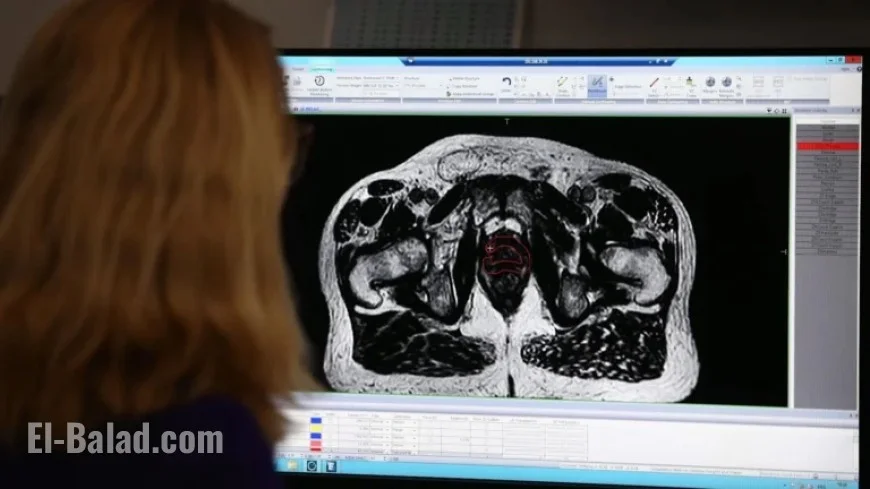
A new clinical trial has been launched in the UK to evaluate a prostate cancer treatment known for its reduced side effects. Researchers hope Aquablation, a robotic-assisted therapy, will provide an effective alternative to traditional surgical approaches.
Overview of Aquablation Therapy
Aquablation utilizes robotics, artificial intelligence, and real-time imaging to target prostate cancer. This innovative technique aims to minimize complications such as infection, erectile dysfunction, and urinary problems, which are commonly associated with radical prostatectomy. The procedure is suitable for patients whose cancer has not spread beyond the prostate or has only locally metastasized.
Trial Details
Backed by the National Institute for Health and Care Research (NIHR), this trial involves recruiting 280 patients across seven countries. In the UK, four centers will participate, including:
- The Royal Marsden NHS Foundation Trust
- Guy’s and St Thomas’ NHS Foundation Trust
- Royal Free London NHS Foundation Trust
- Norfolk and Norwich University Hospitals NHS Foundation Trust
The Royal Marsden became the first hospital in Europe to enroll a patient in this pivotal study.
Expert Insights
Philip Charlesworth, a consultant urological surgeon at the Royal Marsden, expressed enthusiasm about the trial’s potential. He stated, “This trial is measuring Aquablation therapy to preserve a man’s ability to remain continent and maintain sexual activity.” He emphasized the importance of reducing the adverse effects of prostate cancer treatments and enhancing patient quality of life.
Background on Prostate Cancer Treatments
Current treatment options for localized prostate cancer include active surveillance and radiation therapy. However, radical prostatectomy remains a prevalent option, although it involves higher risks of complications.
Screening and Health Policies
The trial coincides with recent discussions surrounding prostate cancer screening guidelines. Health Secretary Wes Streeting expressed surprise at the recommendation from the UK National Screening Committee against routine screening for most men. While the committee suggests only screening men with BRCA1 and BRCA2 mutations, discussions on data and alternative screening methods continue.
Experts are awaiting results from a substantial trial by Prostate Cancer UK that may support broader screening recommendations. The debate highlights the complexities surrounding the reliability of PSA testing for prostate cancer detection.
Conclusion
The Aquablation trial represents a significant step towards improving prostate cancer treatment while addressing concerns about side effects. As research progresses, the potential for new therapeutic options may enhance patient outcomes across the globe.