Novo Nordisk Sues Hims Over FDA-Controversial $49 Weight-Loss Pill

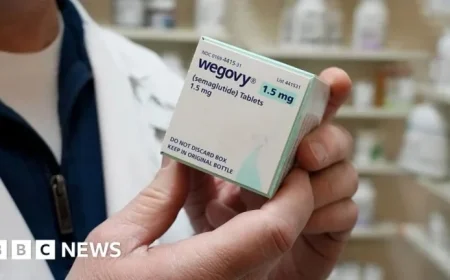
Novo Nordisk has initiated legal action against Hims and Hers Health, alleging patent infringement regarding a controversial weight-loss pill. This lawsuit follows Hims’ brief launch of a $49 version of Novo’s Wegovy, which sparked regulatory backlash in the United States.
Details of the Lawsuit
The lawsuit, filed in a U.S. court, addresses issues surrounding both the pill and injectable forms of Novo’s weight-loss drug. Novo claims that Hims’ products infringe upon its patents, and it is seeking a permanent injunction to prevent further sales.
Market Reaction
- Novo Nordisk’s stock rose by 5% after the lawsuit announcement.
- Conversely, Hims’ shares dropped sharply by 20%.
- Both Novo and Eli Lilly had experienced stock declines prior to the legal action.
The Broader Context
John Kuckelman, Novo’s general counsel, emphasized that this lawsuit represents a significant moment for the pharmaceutical industry. Hims described this legal move as a direct attack on the accessibility of compounded medications for many Americans.
FDA’s Role
The U.S. Food and Drug Administration (FDA) recently stated its intention to restrict GLP-1 ingredients used in compounded drugs, adding pressure to the market for these weight-loss treatments.
Concerns for the Future
Industry analysts suggest that Novo’s legal strategy could signify a push to curb competition from compounders offering similar products. This lawsuit against Hims marks a shift from Novo’s traditional approach, focusing on ensuring its patents are not violated.
Competition and Market Dynamics
- Novo’s market value has decreased nearly 50% over the last year.
- The introduction of Eli Lilly’s oral GLP-1 pill is anticipated to heighten competition further.
- Both companies are navigating a market shift towards direct-to-consumer models.
With significant investments and partnerships, including past pricing arrangements with the Trump administration, both Novo and Lilly are trying to secure their positions in a rapidly evolving market. The outcome of this lawsuit could have lasting implications for the accessibility of compounded medications and the strategies of major pharmaceutical companies.