mRNA Vaccines Enhance Tumor Sensitivity to Immune Checkpoint Blockade
Recent research suggests that mRNA vaccines may enhance tumor sensitivity to immune checkpoint blockade in cancer treatments. This study involved a comprehensive retrospective analysis of patient data from the MD Anderson Cancer Center (MDACC) in Houston, Texas. The review focused on patients with advanced non-small cell lung cancer (NSCLC), melanoma, and a tissue-agnostic cohort.
Study Overview
The analysis included three distinct groups of patients:
- Patients with stage III or IV NSCLC treated between January 2017 and September 2022.
- Patients with melanoma receiving immune checkpoint inhibitors from January 2019 to December 2022.
- A tissue-agnostic cohort recorded for PD-L1 pathology results from January 2020 to October 2023.
The Institutional Review Board at MD Anderson approved the study, and informed consent was waived because of the retrospective nature of the data.
Patient Data Collection
Data collected included demographics such as age, sex, and ethnicity, along with details on tumor histology, stage, mutations (e.g., EGFR, KRAS, ALK), and treatment history. Specific COVID-19 vaccination records were also documented, given the potential intersection with individual cancer treatments.
mRNA Vaccination and Immune Response
COVID-19 mRNA vaccine formulations included both monovalent and bivalent types from Moderna and Pfizer/BioNTech. Patients were categorized into two groups based on vaccination status within 100 days of starting immune checkpoint inhibitors:
- Those who received an mRNA vaccine.
- Those who did not.
Survival Analysis
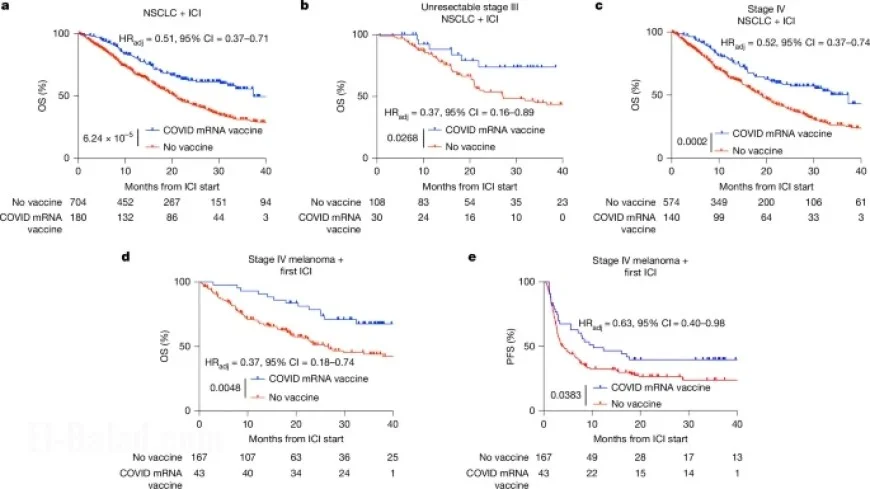
Overall Survival (OS) and Progression-Free Survival (PFS) were calculated based on treatment initiation relative to vaccination dates. The analysis indicated a significant improvement in OS and PFS for patients who received vaccinations close to the initiation of their cancer treatments.
Cox Proportional Hazards Model
The study employed the Cox proportional hazards regression model to examine variables influencing survival rates. Patient characteristics, including treatment history and PD-L1 expression, were analyzed to determine their impact on outcomes.
Statistical Findings
The results were visualized using Kaplan–Meier curves, which indicated better survival rates in patients responding to the combination of mRNA vaccination and immune checkpoint therapy. Statistical analysis confirmed significant correlations between mRNA vaccine administration and improved patient outcomes.
Conclusion
This research underscores the potential of mRNA vaccines to enhance the efficacy of immune checkpoint inhibitors in cancer therapy. Further studies are necessary to fully understand the mechanisms at play and to optimize treatment strategies for better patient care.