Atorvastatin recall: 141,984 bottles pulled nationwide over “failed dissolution” concerns
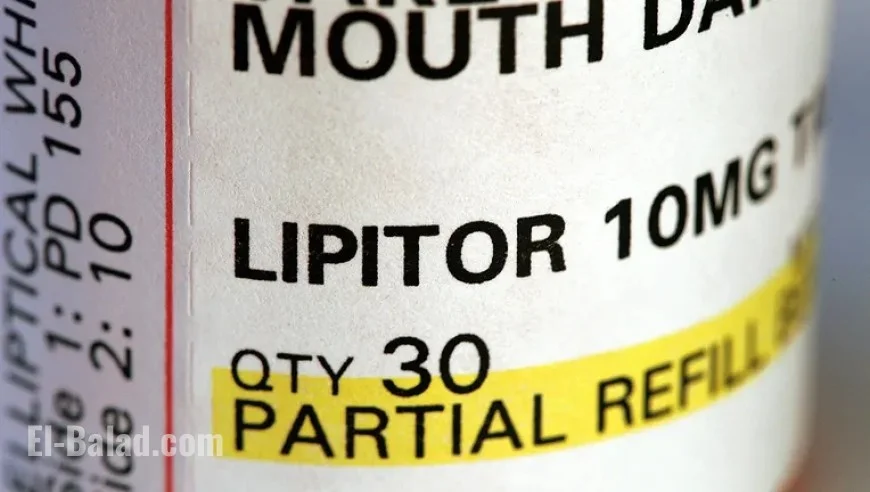
A nationwide atorvastatin recall is underway after quality testing flagged tablets that may not dissolve properly—potentially lowering effectiveness for some users. The action, covering 141,984 bottles of prescription atorvastatin calcium (generic for Lipitor), has been classified as a Class II recall, indicating the risk of serious harm is remote but that temporary or medically reversible effects are possible. The products were manufactured by Alkem Laboratories and distributed by Ascend Laboratories across the U.S.
What the atorvastatin recall includes
-
Dosages affected: 10 mg, 20 mg, 40 mg, and 80 mg tablets
-
Bottle sizes: 90-count, 500-count, and 1,000-count
-
Scope: Eight specific lot numbers, with expiration dates ranging from July 2026 to February 2027
-
Reason: Failed dissolution specifications (tablets may not dissolve at the intended rate during testing, which can reduce how well the medicine works)
-
Regulatory status: Class II recall; products are prescription-only and were distributed nationwide
-
Timeline: Recall initiated in September and formally classified as Class II on October 10, 2025
Dissolution failures are a quality/efficacy issue—not evidence of contamination. The primary concern is that the drug could deliver less cholesterol-lowering effect than intended.
Examples of identifiers you may see on bottles
-
NDCs commonly cited for 10 mg: 67877-511-90 (90 ct), 67877-511-05 (500 ct), 67877-511-10 (1,000 ct)
-
Sample lot codes seen in recall notices (10 mg): 25141249 (Exp. Feb 2027), 24144938, 24144868, 24144867 (Exp. Nov 2026)
(Other strengths have their own lots; check your label.)
What patients should do now
-
Do not stop taking atorvastatin on your own. Sudden discontinuation can raise cardiovascular risk.
-
Check your bottle label for the strength, NDC, and lot number. If it matches a recalled lot, contact your pharmacy first—they can confirm lot status and arrange a replacement.
-
Speak with your prescriber about whether to repeat a lipid panel sooner than planned if you’ve been on a recalled lot, especially if you have high-risk cardiovascular disease.
-
Store and return: Keep tablets in the original container until the pharmacy instructs you on return or disposal procedures for recalled medicine.
-
Report issues: If you’ve noticed unexpected changes in cholesterol results or any adverse effects, tell your clinician and consider submitting a report to the appropriate safety program.
Guidance for pharmacists and clinics
-
Quarantine affected stock immediately and follow the recall’s retail-level instructions.
-
Notify patients who received impacted lots when possible; offer equivalent replacement or coordinate with prescribers for an alternative statin.
-
Document actions for state board and payer audits (date of quarantine, lot reconciliation, patient outreach).
-
Counseling point: Emphasize that the recall is not about contamination; it’s a performance issue that may blunt LDL-lowering. Reassure patients while expediting swaps.
Why this atorvastatin recall matters
Atorvastatin is one of the most prescribed statins. For higher-risk patients, even a modest drop in potency can influence LDL-C targets and, over time, cardiovascular outcomes. While a Class II classification signals low likelihood of serious harm, it warrants prompt verification of your supply and continuity of therapy without gaps.
Key dates and details at a glance
| Item | Detail |
|---|---|
| Recall classification | Class II (quality issue; serious harm unlikely) |
| Products | Atorvastatin calcium 10/20/40/80 mg |
| Bottle sizes | 90, 500, 1,000 tablets |
| Lots | Eight lots (check bottle label) |
| Expirations | Jul 2026 – Feb 2027 |
| Manufacturer / Distributor | Alkem Laboratories / Ascend Laboratories |
| Nationwide distribution | Yes |
| Action window | Initiated September; classified Oct 10, 2025 |
Frequently asked questions
Is it dangerous to take these tablets?
The issue involves dissolution, not contamination. The main risk is reduced efficacy. Keep taking your medicine until your pharmacist or prescriber advises a switch.
Will insurance cover a replacement?
In recall situations, pharmacies typically provide a no-cost replacement of the same drug/strength from unaffected lots or coordinate with your prescriber for an alternative covered option.
Should I switch statins?
Not necessarily. Many patients can continue atorvastatin from unaffected lots. If supply is constrained or you’ve had LDL-C setbacks, your clinician may discuss alternatives (e.g., rosuvastatin) or temporary dose adjustments.
The current atorvastatin recall is large but targeted to specific lots tied to a dissolution problem. Don’t stop your statin abruptly; verify your lot, work with your pharmacist for a replacement if needed, and stay on therapy to protect your heart health.