FDA Approvals for 2025 Await Key Innovations
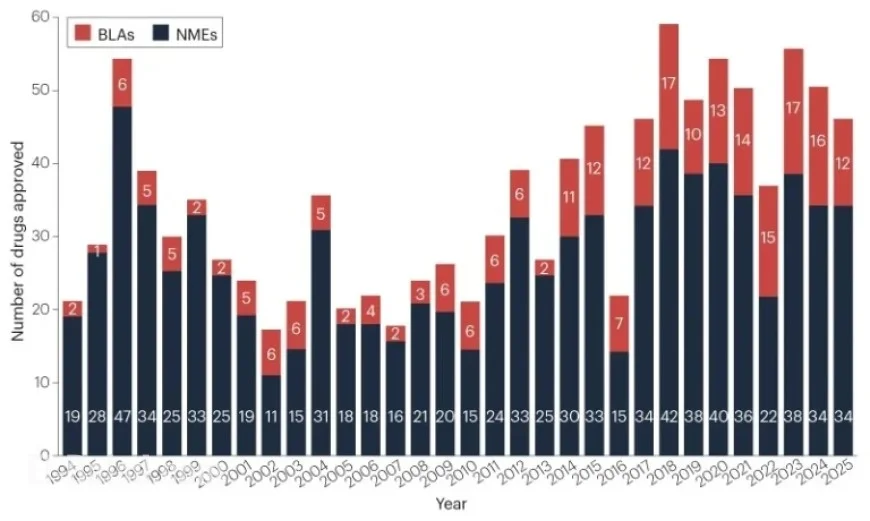
The FDA’s Center for Drug Evaluation and Research (CDER) approved 46 new therapeutic agents in 2025. This brought the five-year average of new drug approvals down slightly to 48 annually, yet still reflects a healthy pipeline above the historical average of 36 since 1993.
FDA Approvals for 2025 Highlight Key Innovations
Among the approved drugs, cancer therapies dominated, representing 35% of new approvals. This is an increase from a 29% average over the past five years. In total, 16 new drugs targeted cancer, while cardiology saw 5 new approvals, and allergy and inflammatory diseases accounted for 4 new drugs.
Growth in Innovative Drug Modalities
The year 2025 marked a significant expansion in drug modalities. Notably, a first adnectin-based biologic therapy received approval. Additionally, kinase inhibitors rose in prominence, with one-third of the new small molecules being categorized as this type of therapy. The landmark approval for Novartis’s remibrutinib marked its 100th kinase inhibitor.
Major Developments in Cancer Therapies
- Merck’s pembrolizumab (Keytruda Qlex), now in a subcutaneous formulation, is projected to generate $32 billion in sales.
- Two antibody-drug conjugates (ADCs) received approval for cancer treatment, including Daiichi Sankyo’s datopotamab deruxtecan.
Challenges Within the FDA
Despite the successes, 2025 was turbulent for the FDA. Over 18% of CDER and CBER staff left their positions due to dramatic policy shifts. The agency saw five leadership changes and implemented new approval pathways, such as the FDA Commissioner’s National Priority Voucher (CNPV) pilot program, aiming to significantly reduce review times.
Highlights in Non-Oncology Approvals
Non-oncology drug approvals included pivotal advancements for various chronic conditions:
- Brensocatib (Brinsupri): The first DPP1 inhibitor for bronchiectasis, showing peak sales potential of $6.3 billion.
- Suzetrigine (Journavx): A non-opioid painkiller targeting the NaV1.8 sodium channel with expected sales of $3.7 billion.
- Gepotidacin (Blujepa): A new oral antibiotic for urinary tract infections, highlighting a fresh approach in treatment.
Insights into Futures Approvals
Looking ahead, drug developers are poised to introduce groundbreaking therapies. Arvinas’s vepdegestrant, a potential first-in-class targeted-protein degrader, and Denali Therapeutics’s enzyme replacement therapy, tividenofusp alfa, are among the contenders for FDA approval in the near future.
The continuous improvements in drug development and approval processes underscore the FDA’s commitment to addressing unmet medical needs through innovation. With a strong focus on regulatory advancements and diverse therapeutic modalities, the pharmaceutical landscape is evolving rapidly.







































