RNA Sequestration in Biomolecular Condensates Guides Cell Fate Changes
Recent research has shed light on the role of RNA sequestration in biomolecular condensates, particularly in the context of P-bodies. These cellular structures are pivotal in determining cell fate changes, especially during the differentiation of stem cells. The findings emphasize how specific RNA molecules are stored within P-bodies to regulate protein synthesis and influence developmental pathways.
Profiling P-body Contents
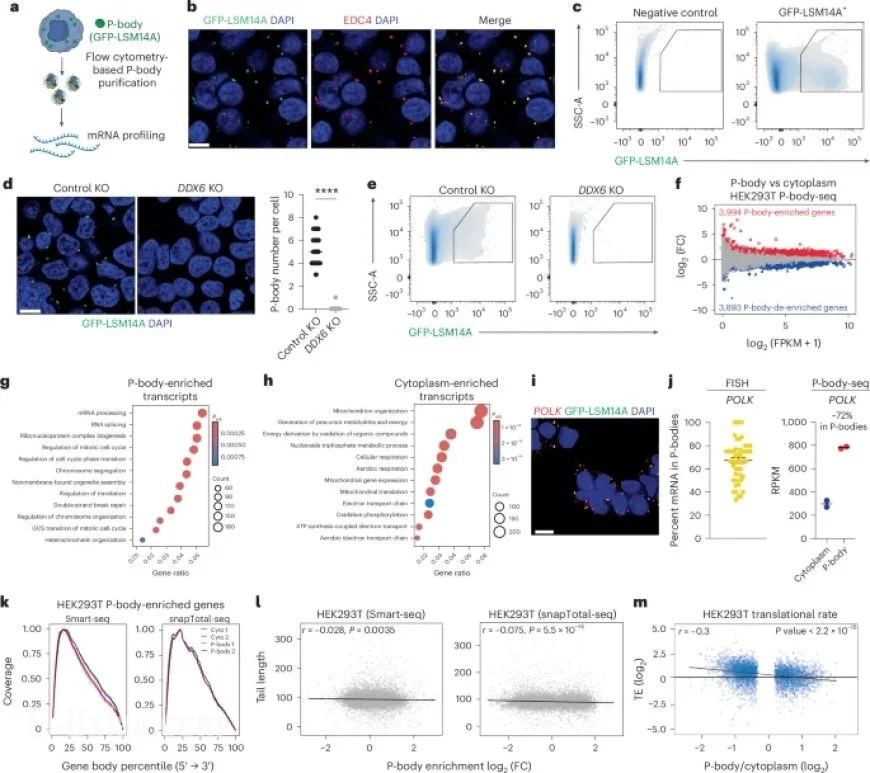
The study began by employing advanced sorting techniques to analyze P-body contents in human embryonic kidney cells (HEK293T). Researchers utilized a GFP-tagged version of the LSM14A protein, a known component of P-bodies, to isolate and profile RNA sequences from these cellular structures. The methodology allowed for visual confirmation of P-body integrity prior to isolation, using fluorescence-activated particle sorting (FAPS).
RNA Presence in P-bodies
Through various assays, researchers confirmed that RNA is indeed present in GFP-LSM14A particles, while particles from control cells lacking this specific construct did not show detectable RNA. This validation underscores the effectiveness of the isolation method and sets the stage for subsequent analyses.
P-body Enrichment and Cell Fate Regulation
The study detected nearly 4,000 mRNAs that were significantly enriched within P-bodies when compared to cytoplasmic RNA, indicating a clear involvement in regulatory processes like transcription and cell cycle management. Gene ontology (GO) analyses reinforced the idea that P-bodies play a crucial role in buffering cellular responses during differentiation.
Influence of P-bodies on Cell Differentiation
Further investigations revealed that the RNA contents of P-bodies change depending on the cell type and their developmental stage. For instance, human embryonic stem cells (hESCs) exhibited distinct P-body profiles when differentiated into progenitor cells of various lineages. This variability in P-body content highlights a selective sequestration mechanism that likely helps maintain pluripotency and regulates the timing of differentiation.
Mechanisms of RNA Sequestration
Understanding how RNA is directed into P-bodies is essential. The study explored the roles of microRNAs (miRNAs), which were found to target specific mRNAs for sequestration in P-bodies. Disruption of miRNA function resulted in altered localization of mRNA, leading to changes in protein expression and cellular identity.
Functional Implications of P-body Disruption
By disabling P-bodies through targeted gene editing, researchers demonstrated an increase in the translation of previously sequestered mRNAs, particularly those involved in establishing a 2C-like state, a transient totipotent state in mouse embryonic stem cells. This suggests that P-bodies not only act as storage sites but also play an active role in modulating the operational landscape of gene expression during development.
Conservation Across Species
A critical aspect of the findings is the conservation of P-body functions and RNA sequestration mechanisms across vertebrate species. Both human and mouse P-bodies were shown to share common mRNA profiles, particularly those associated with embryonic development and stem cell maintenance, suggesting a fundamental evolutionary significance.
Applications in Stem Cell Engineering
The implications of these findings extend to stem cell engineering and regenerative medicine. Manipulating P-body dynamics offers a promising strategy for enhancing stem cell potency and directing lineage-specific differentiation, particularly in eliciting the transition to germ cell-like states. Researchers believe that targeting P-body components could eventually lead to improved methods for generating rare cell types in vitro.
Conclusion
In summary, RNA sequestration in P-bodies is not a passive process but a dynamic mechanism that guides cell fate decisions during development. Continued exploration of these biomolecular condensates may offer new insights into stem cell biology and therapeutic interventions for regenerative medicine.