Abbott Recalls 3 Million Glucose Sensors Following Seven Deaths

Abbott Diabetes Care has initiated a recall of approximately 3 million glucose sensors due to safety concerns. This decision comes after reports of seven fatalities and over 700 injuries linked to malfunctioning devices that may deliver inaccurate glucose readings.
Recall Details and Affected Products
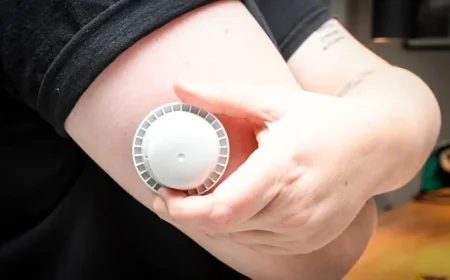
The U.S. Food and Drug Administration (FDA) announced the recall on December 2. Abbott’s FreeStyle Libre 3 and FreeStyle Libre 3 Plus devices are the main products affected. Internal tests revealed that these sensors might produce inaccurate low glucose readings.
- Product Affected: FreeStyle Libre 3
- Product Affected: FreeStyle Libre 3 Plus
- Total Sensors Recalled: Approximately 3 million
Half of these sensors have already been used or expired. The recall specifically involves sensors from a single production line. Notably, no other Libre products are implicated in this recall.
Health Risks Associated with Malfunctioning Sensors
Reports indicate that Abbott received 736 severe adverse events potentially tied to these monitors, with 57 incidents occurring in the U.S. The seven reported deaths happened outside the country. The FDA has categorized this issue as “potentially high-risk.”
“If left unaddressed, incorrect low glucose readings could result in improper treatment decisions. This may include excessive carbohydrate consumption or inappropriate insulin dosage adjustments,” Abbott stated. Such errors could lead to serious health consequences, including injury or death.
Recommendations for Affected Users
Both Abbott and the FDA advise users of the affected sensors to cease usage immediately. Patients should rely on a blood glucose meter or the built-in meter on a FreeStyle Libre 3 Reader for making treatment decisions, especially if sensor readings are inconsistent with their symptoms.
How to Check If Your Sensor Is Affected
Individuals can verify if their sensor is part of the recall by visiting www.FreeStyleCheck.com. They can enter the model number and the unique device identifier of their device. The model numbers and identifiers for affected products are listed below:
| Device Type | Model Number | Unique Device Identifier |
|---|---|---|
| FreeStyle Libre 3 | 72081-01 | 00357599818005 |
| FreeStyle Libre 3 | 72080-01 | 00357599819002 |
| FreeStyle Libre 3 Plus | 78768-01 | 00357599844011 |
| FreeStyle Libre 3 Plus | 78769-01 | 00357599843014 |
Next Steps for Abbott
Abbott is providing replacements for all affected sensors at no charge. The company has identified and rectified the issues leading to the malfunctions and does not expect any major supply chain disruptions for future orders.